
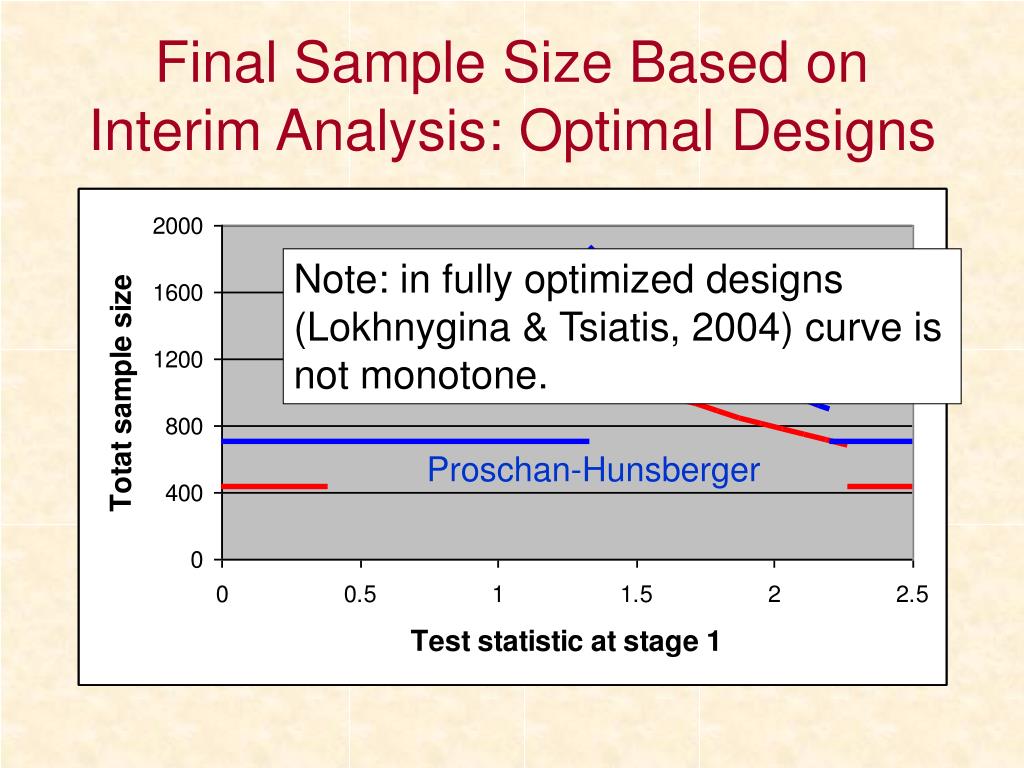
The following shows the possible sampling outcomes and the preferences for test decision outcome. It is based on the work of Abraham Wald (Ref. This tool provides the ability to plan a sequential lot acceptance test where each unit is classified into one of two categories, good or defective. 2008 13(1): 31 - 40.īACANLI S,PEKER Ö,DEMİRHAN Y "Two-stage group sequential designs for comparative clinical trials." Mathematical and Computational Applications, 13, ss.31 - 40, 2008.Sequential Testing: Testing the Mean of a Binomial Distribution Mathematical and Computational Applications. Mathematical and Computational Applications, vol.13, no.1, 2008, ss.31 - 40.īACANLI S,PEKER Ö,DEMİRHAN Y Two-stage group sequential designs for comparative clinical trials. Mathematical and Computational Applications 13, no.1 (2008): 31 - 40.īACANLI SEVİL,PEKER Özgür K.,DEMİRHAN Yaprak Parlak Two-stage group sequential designs for comparative clinical trials. Mathematical and Computational Applications, 13(1), 31 - 40.īACANLI SEVİL,PEKER Özgür K.,DEMİRHAN Yaprak Parlak Two-stage group sequential designs for comparative clinical trials.

Two-stage group sequential designs for comparative clinical trials. DeMets, Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer, New England Journal of Medicine, 326, 852-856, 1992.īACANLI S, PEKER Ö, DEMİRHAN Y (2008). Peker, S.Bacanlı, Optimal restricted two-stage design, Anadolu University Journal of Science and Technology, 1(1), 95-103. Davis, Three-stage designs for monitoring clinical trials, Communications in Statistics-Theory and Methods, 23 (7), 1875-1893,1994. Lan, Programs for computing group sequential bounds using the Lan-Demets Method, Technical Report, 60, University of Wisconsin Department of Biostatistics, 45 p.1992. Davis, Optimal restricted two-stage designs, Contr. DeWith, Two-stage plans for the testing of binomial parameters, Contr. McPherson, Two-stage plans compared with fixed-sample size and Wald SPRT plans, J. Hald, Optimum double sampling tests of given strength, The normal distribution, J. Owen, A double sample test procedure, Ann. DeMets, Design and analysis of group sequential tests based on type-1 error spending rate function, Biometrika, 74,149-154, 1987. DeMets, Discrete sequential boundaries for clinical trials, Biometrika, 70, 659-663, 1982. Tsiatis.Group sequential designs for one-sided and twosided hypothesis testing with provision for early stopping in favour of the null hypothesis, J.Statist Plan.Infer 42, 19-35, 1994. S.S., Emerson, T.R., Fleming, Symmetric group sequential test design, Biometrics,45, 905-23, 1989. Turnbull, Group Sequential Methods with Application Clinical Trials, Chapman and Hall/ Boca Raton, 2000. Jennison, Optimal asymmetric one-sided group sequential tests, Biometrika, 89, 49-60, 2002. Jennison, An improved method for deriving optimal one-sided group sequential tests, Biometrika, 79, 13-24,1992. C.Jennison, Efficient group sequential test with unpredictable group sizes, Biometrika, 74 155-65, 1987. Ware, Asycmetric group sequential boundaries for monitoring clinical trial, Biometrika, 69, 661-663, 1982. Ware, Group sequential methods for clinical trials with a onesided hypothesis, Biometrika, 67, 651-60, 1980. Armitage, Sequential medical trials, 2nd ed. P.C.O’Brien, T.R.Fleming, A multiple testing procedure for clinical trials. Pocock, Group sequential methods in the design and analysis of clinical trials,Biometrika, 64, 191-199, 1977. DeMets, Sample size determination for sequential clinical trials with immediate response, Statistics in Medicine, 11, 1391-1399,1992.


 0 kommentar(er)
0 kommentar(er)
